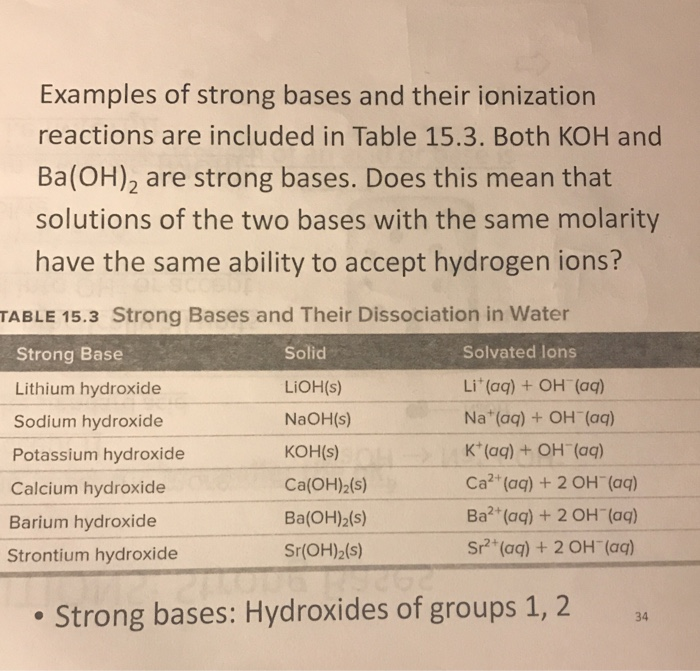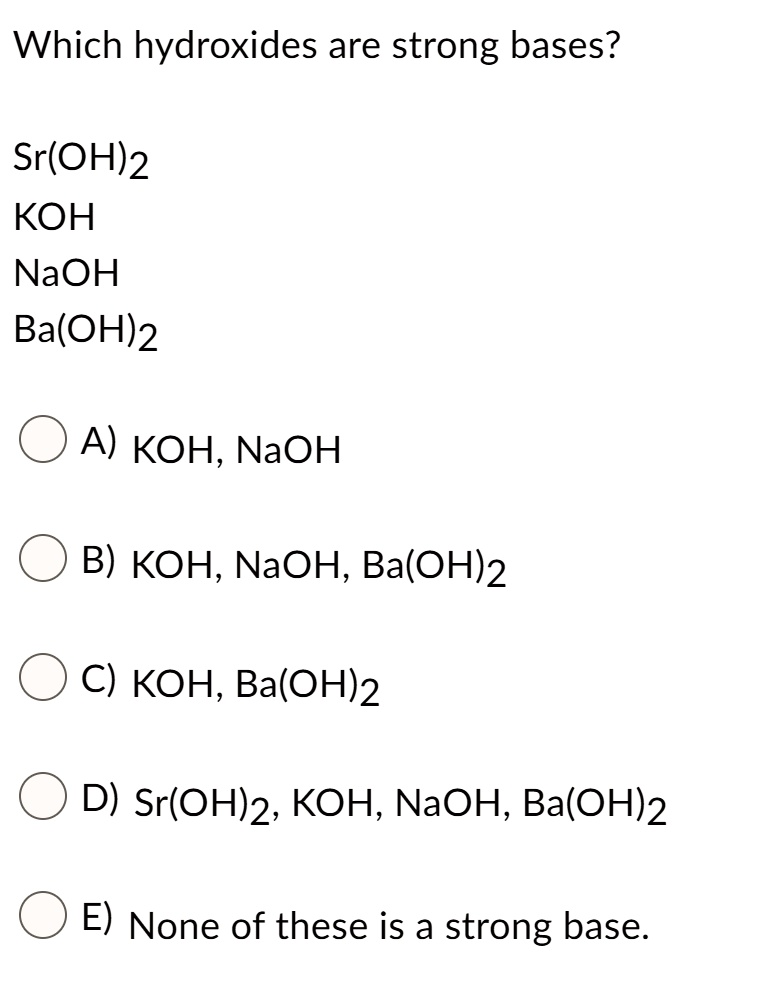
SOLVED: Which hydroxides are strong bases? Sr(OH)2 KOH NaOH Ba(OH)2 A) KOH, NaOH B) KOH, NaOH; Ba(OH)2 C) KOH, Ba(OH)2 D) Sr(OHJ2, KOH; NaOH, Ba(OH)2 E) None of these is a strong
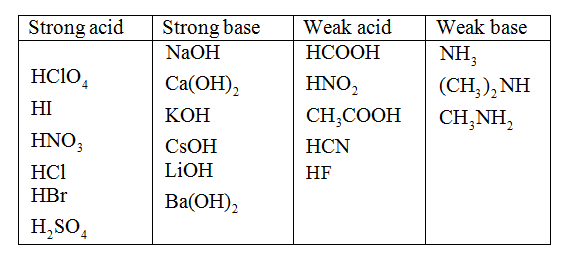
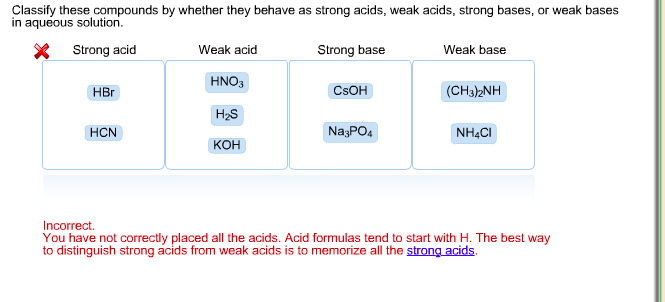
Classify each substance as a strong acid, strong base, weak acid, or weak base - Home Work Help - Learn CBSE Forum

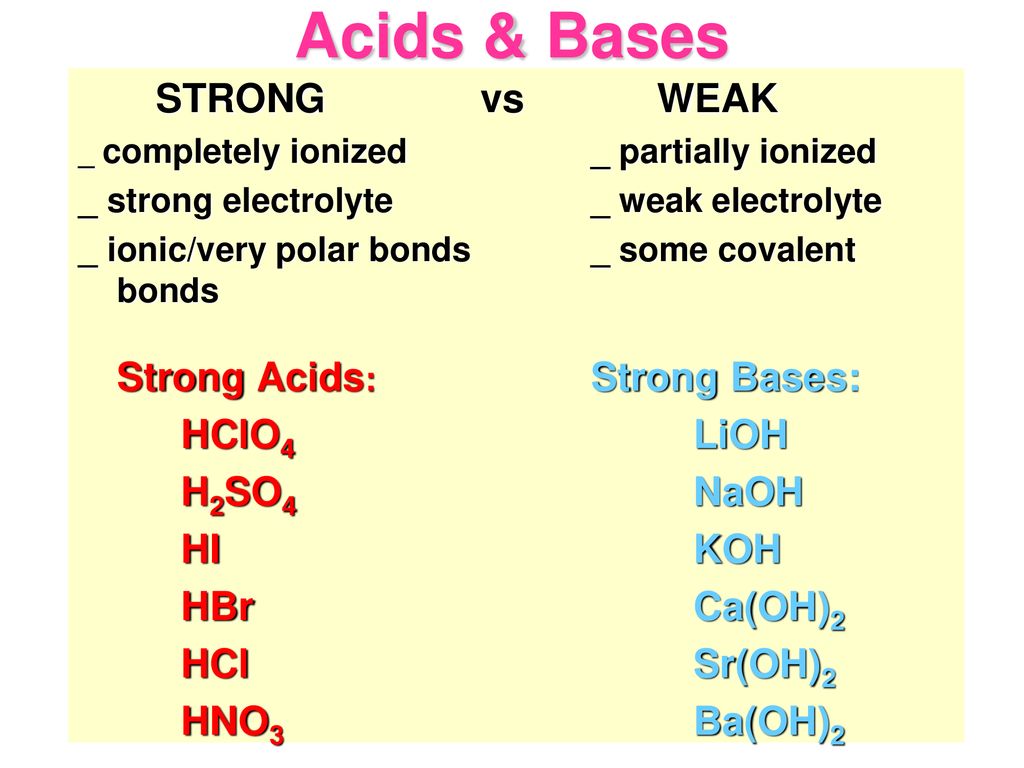
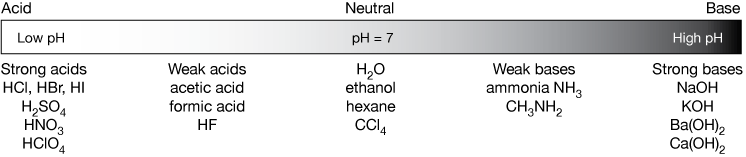
Strengths of Acids and Bases. What does it mean to be strong? In regards to an acid or base: The strength of an acid or base has nothing to do with Molarity. -









:max_bytes(150000):strip_icc()/most-common-strong-bases-603649-ADD-Final2-a2c0ac3120ff4b65bd98989ee298878c.png)