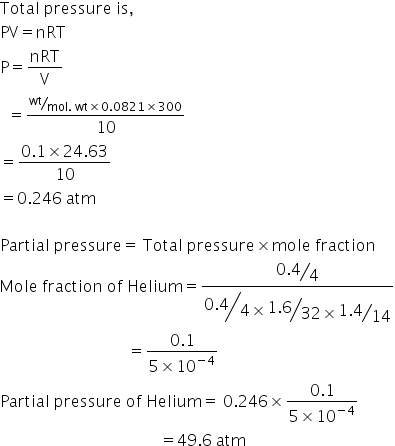
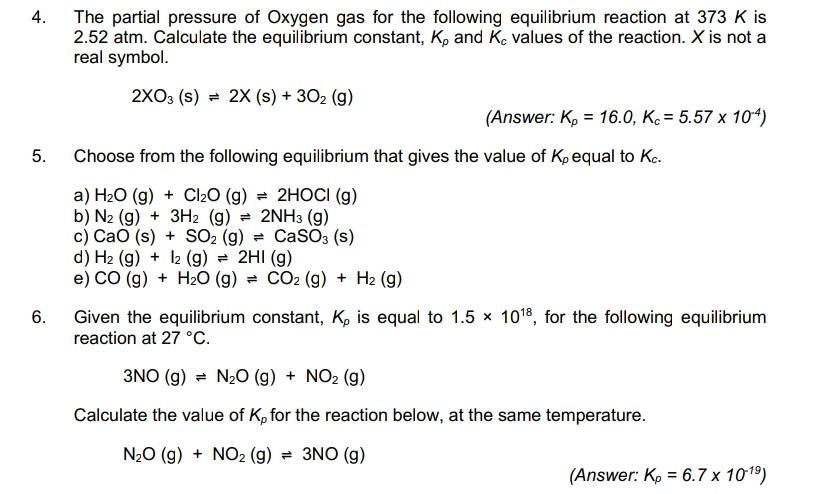
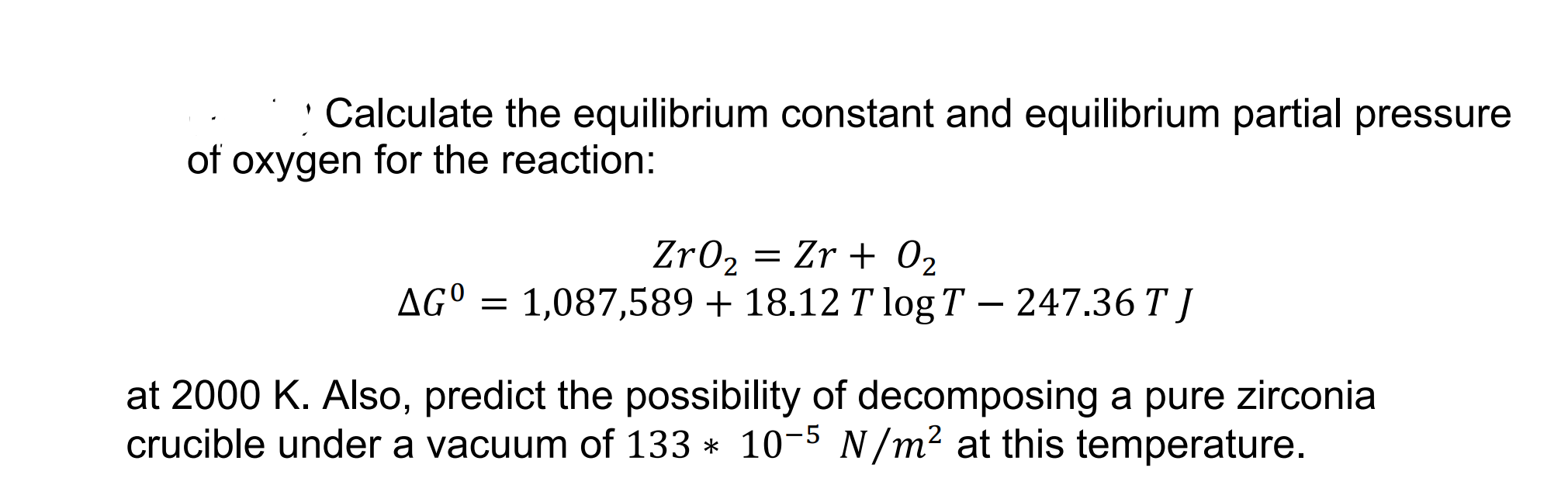
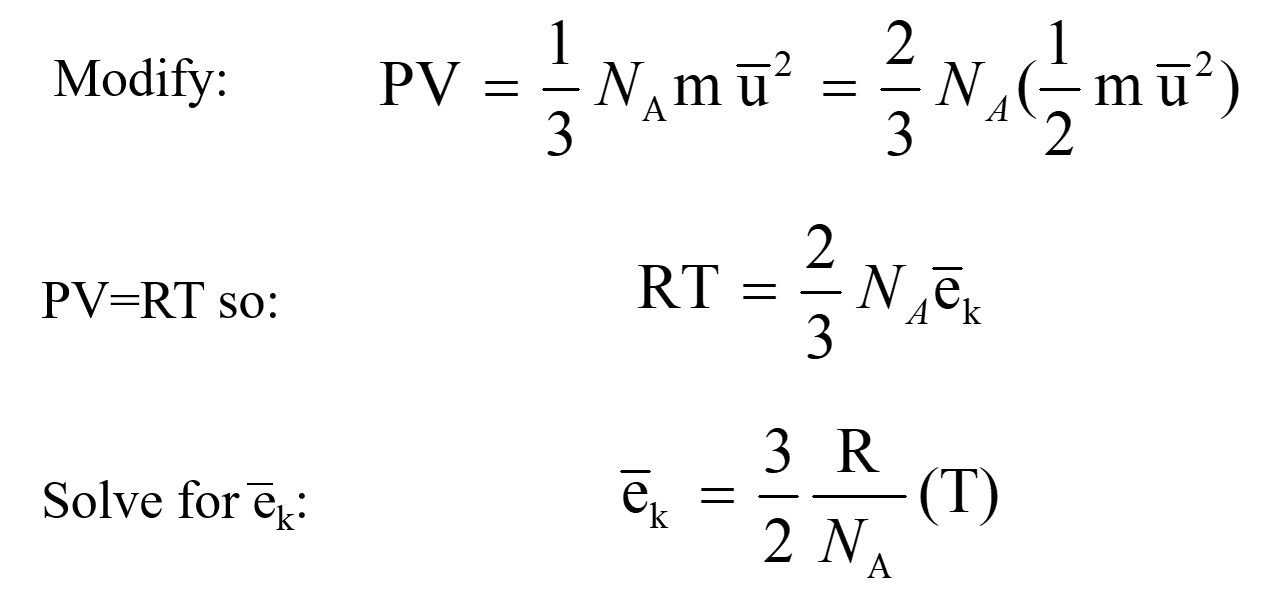OpenStax College Physics Solution, Chapter 13, Problem 62 (Problems & Exercises) | OpenStax College Physics Answers

Dalton's Law of Partial Pressure: Formula | How to Find Partial Pressure - Video & Lesson Transcript | Study.com

SOLVED: The partial pressure of oxygen in the atmosphere is 159. mmHg. Calculate the partial pressure in atm and torr. Round each of your answers to 3 significant digits

SOLVED: Calculate the partial pressure of each of the following gases in room. Air when the barometric pressure is 760 mmHg ( assume the room air contains 21% oxygen, 78% nitrogen, and
![AP chemistry: partial pressures]How do I calculate the partial pressure and the volume of the jar? : r/HomeworkHelp AP chemistry: partial pressures]How do I calculate the partial pressure and the volume of the jar? : r/HomeworkHelp](https://preview.redd.it/4xvi44skj3y51.jpg?auto=webp&s=6c00aa66deba192a88060dcfb5679f4718180123)
AP chemistry: partial pressures]How do I calculate the partial pressure and the volume of the jar? : r/HomeworkHelp
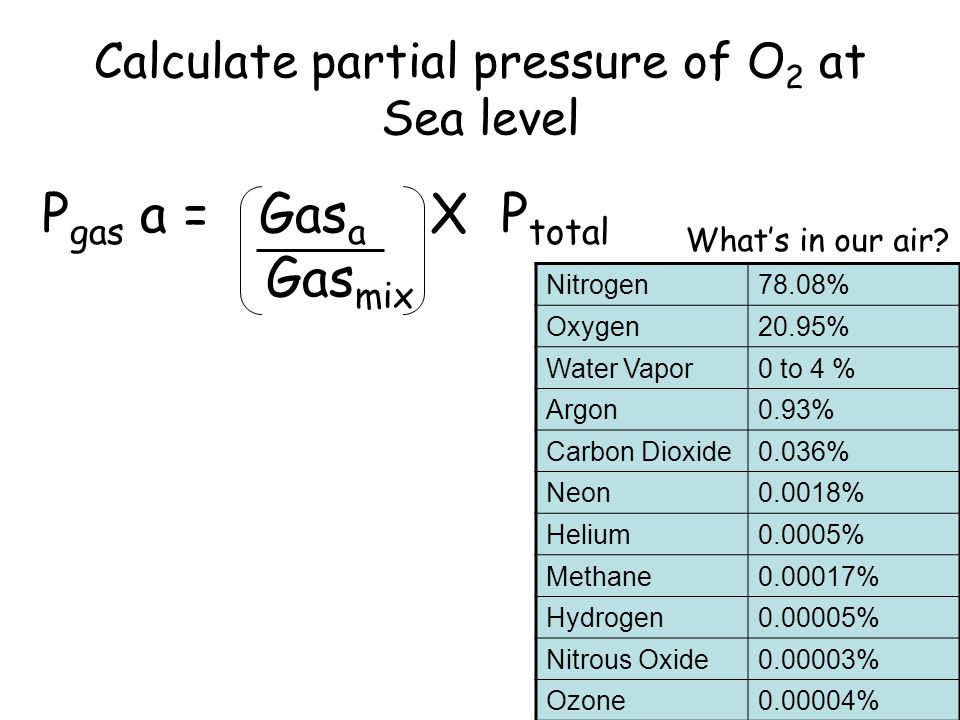
Calculate partial pressure of O 2 at Sea level Nitrogen78.08% Oxygen20.95% Water Vapor0 to 4 % Argon0.93% Carbon Dioxide0.036% Neon0.0018% Helium0.0005% - ppt download