Calculate the molarity and molality of a 13% solution (by weight of sulphuric acid). Its density is 1.090 g/ml.
Welcome to Chem Zipper.com......: Calculate the Molarity of H2O2 if 11.2 ml H2O2 require 30 ml of 0.5 M K2Cr2O7 for its Oxidation . also calculate the volume of strength of H2O2.

I have H2O2 of molecular wt 34.01gm and 30% w/v. What does it mean that I am not getting it and I want to prepare 0.1M solution, how can i? | ResearchGate
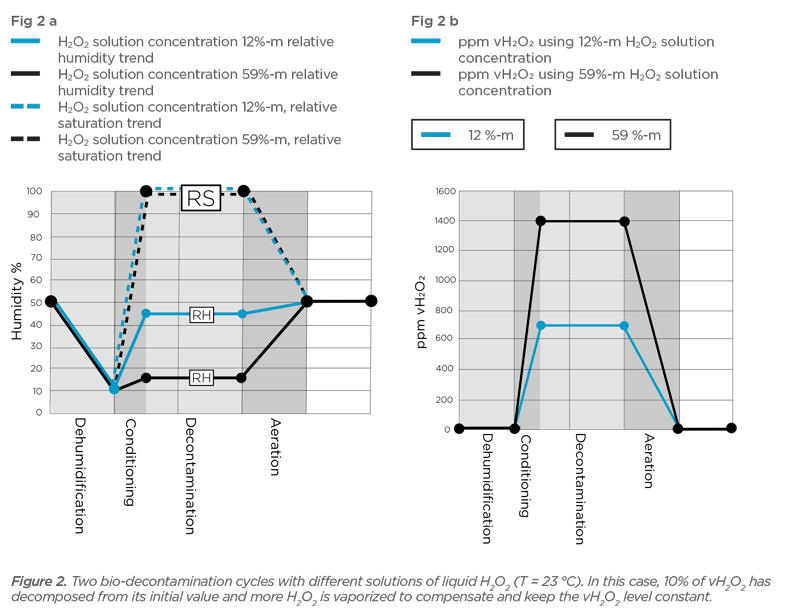
SOLVED: You are required to make up 25.0 ml of a 3.6 M solution of hydrogen peroxide (H2O2). You have at your disposal a stock solution that is 30% by weight, H2O2,
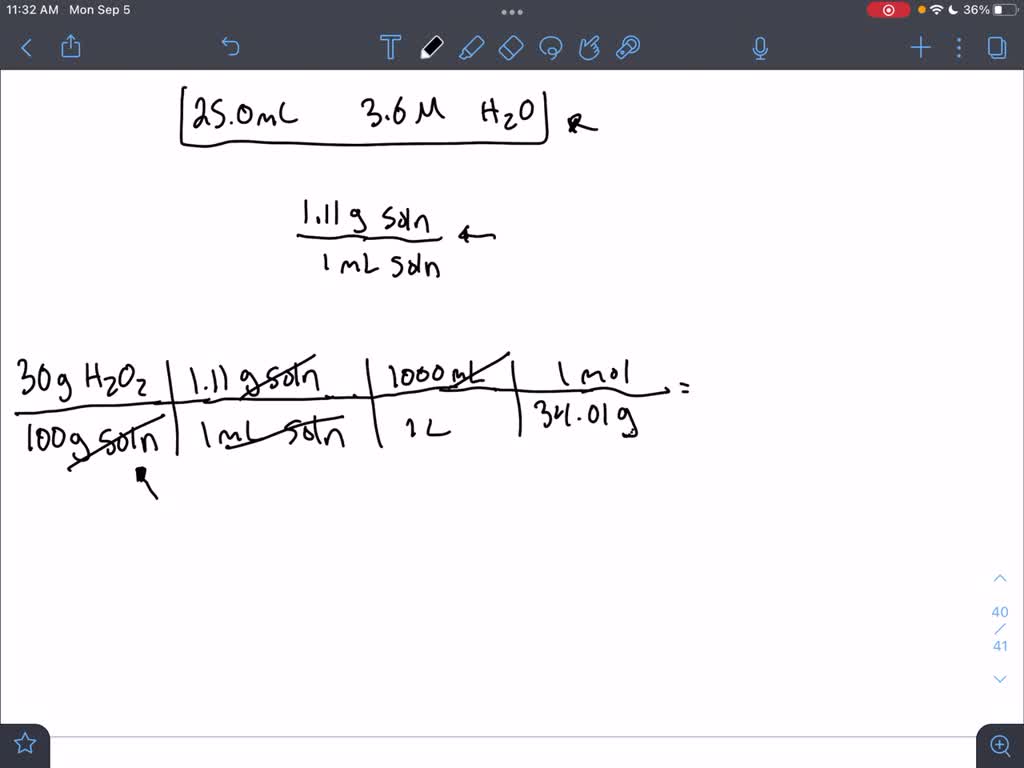
Calculator the equilibrium concentration of gaseous hydrogen peroxide over solution – Lukáš Kolík – physico-chemical calculation – working a personal webpage
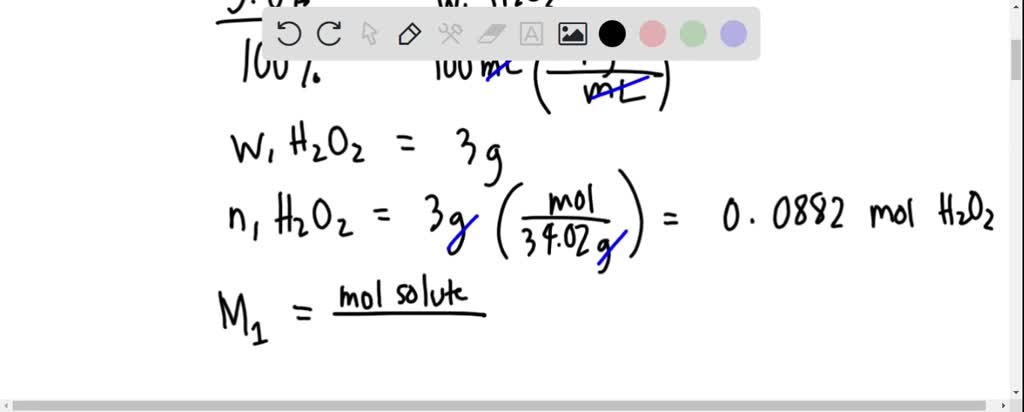
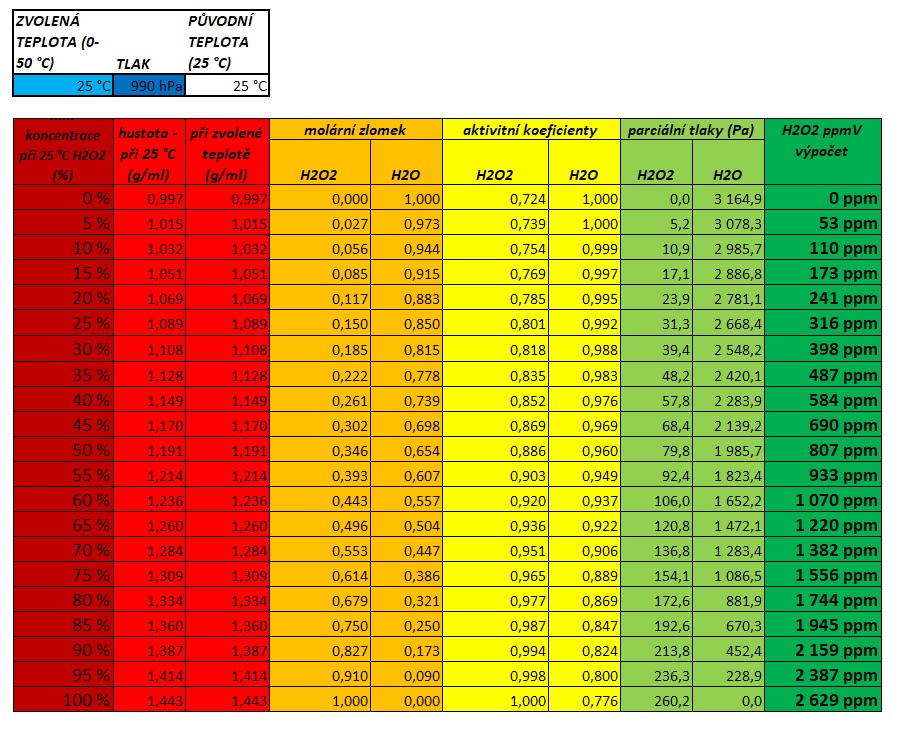
SOLVED: Calculate the final molarity of H2O2 if 5.2 mL of a 3.0% w/w H2O2 solution, which has a density of 1.0 g/mL, is added to 5.2 mL of a starch-iodide solution.


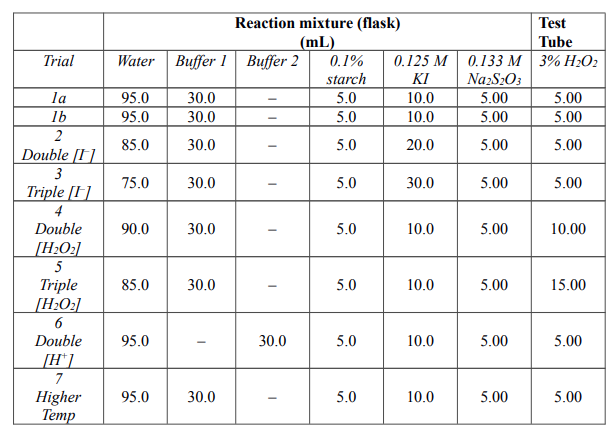





.gif)