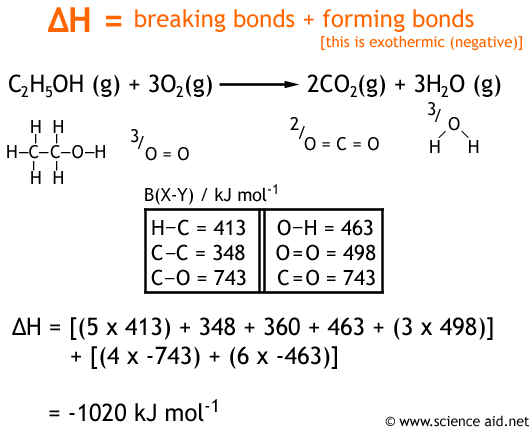
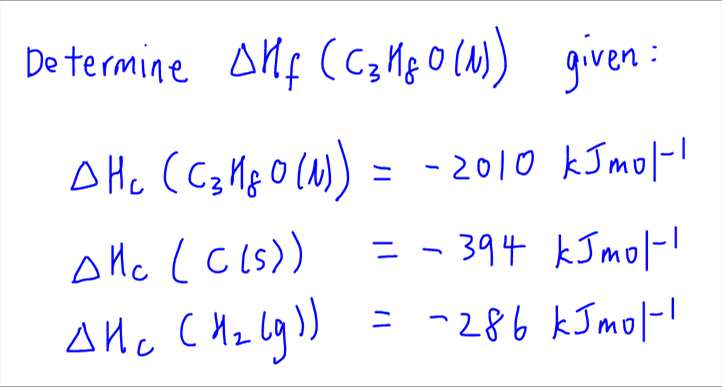Calculate the enthalpy of combustion of ethylene at 1 atm pressure and 298 K, if enthalpy of formation of CO2(g) , H2O(I) and C2H4(g) are - 394, - 242 and - 52 kJ respectively.

Question Video: Calculating the Enthalpy Change for the Reaction between Phenol and Diatomic Hydrogen Using Standard Enthalpies of Combustion | Nagwa
21. Calculate the standard enthalpy of formation of n butane, given that the standard enthalpies of combustion of n butane, C(graphite) and H2(g) are –2878.5 kJ mol–1, –393.5 kJ mol–1 and –285.8

Question Video: Calculating Standard Enthalpy of Combustion of Methane Using Standard Enthalpies of Formation of Methane and Carbon Dioxide | Nagwa
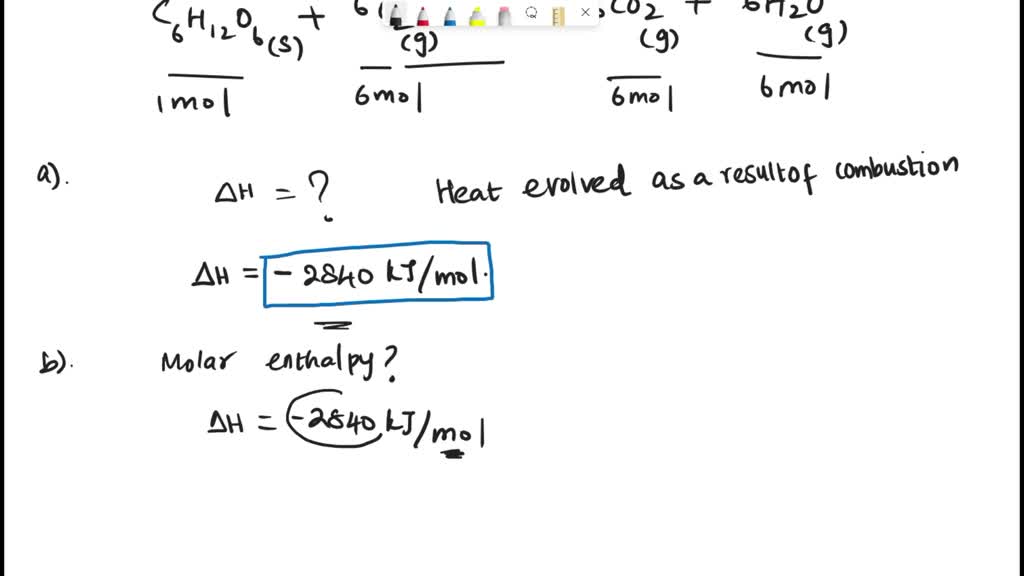
SOLVED: Consider the following reaction equation of combustion of glucose, C6H1206(s) + 6O2(g) -> 6CO2(g) + 6H20(g). a) Calculate enthalpy of combustion of glucose, C6H12O6(s) b) Calculate molar enthalpy of combustion of
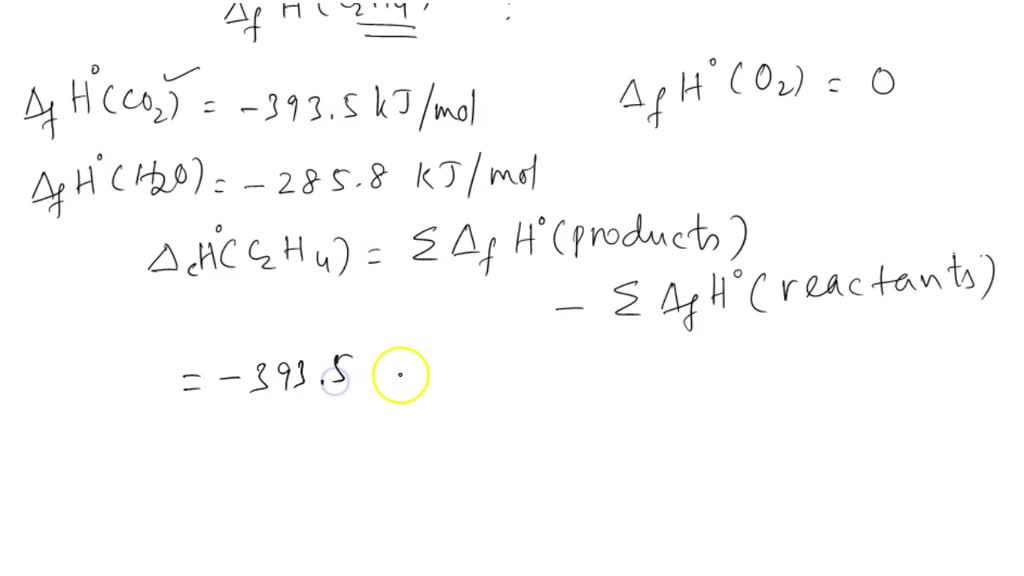
SOLVED: The standard enthalpy of combustion of ethene gas, C2H4 (g), is -1411.1 kJ/mol at 298 K. Given the following enthalpies of formation, calculate ΔHfo for C2H4 (g) CO2 (g): -393.5 kJ/mol