Potassium hydroxide, caustic potash, lye molecule. KOH is strong caustic base and alkali, ionic compound. Structural chemical formula and molecule mod Stock Vector Image & Art - Alamy
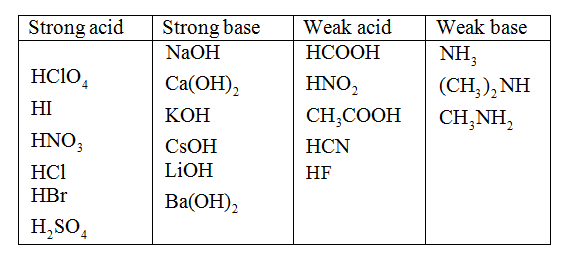
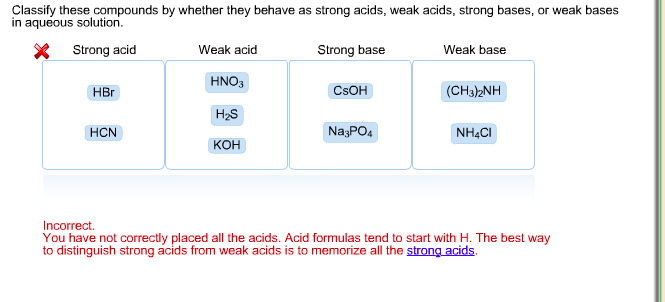
Classify each substance as a strong acid, strong base, weak acid, or weak base - Home Work Help - Learn CBSE Forum

OneClass: Strong acids Weak acids Strong bases Weak bases NaOH KOH HCOOH HNO3 HNO2 CH3NH2 C5H5N C2H C...
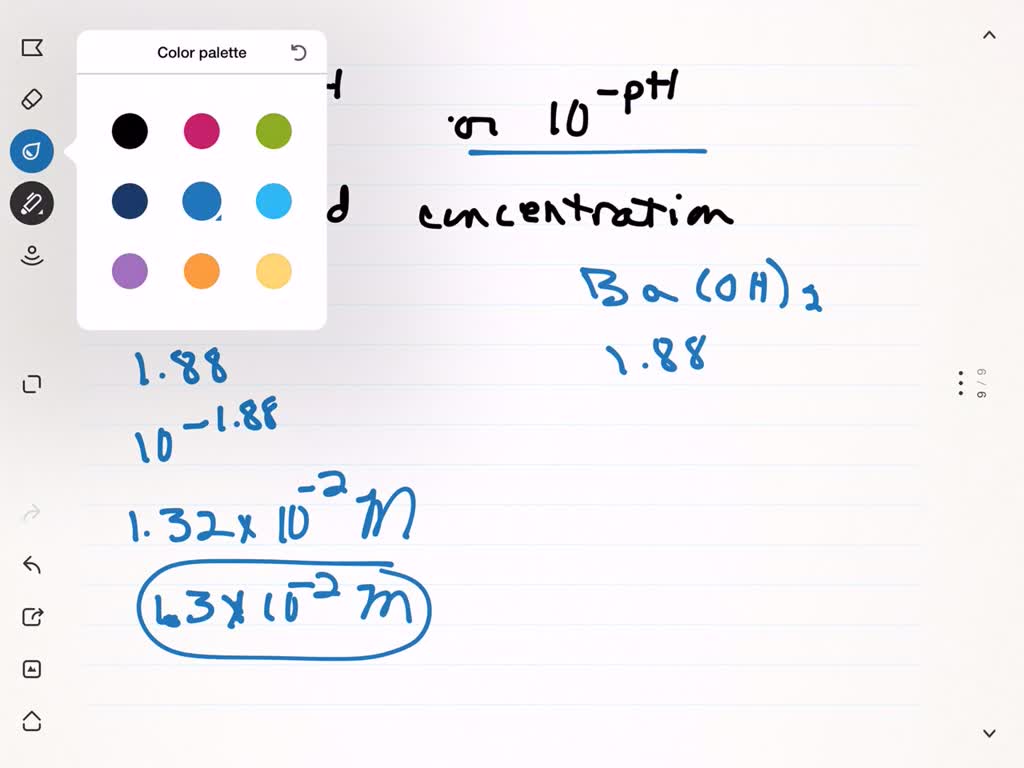
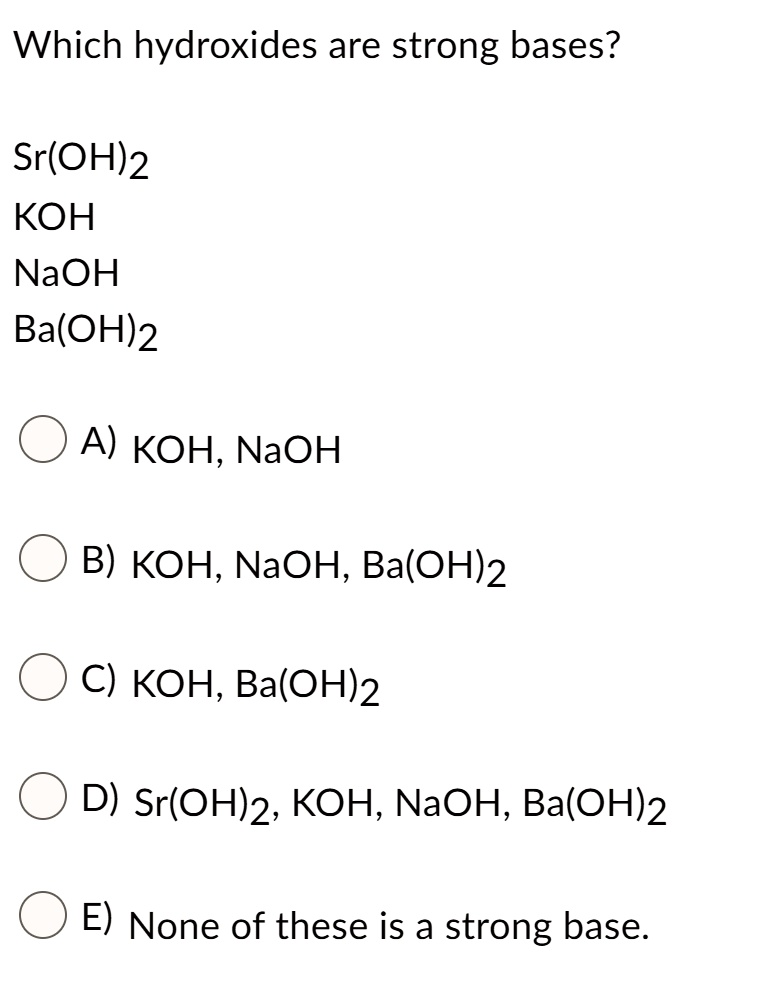
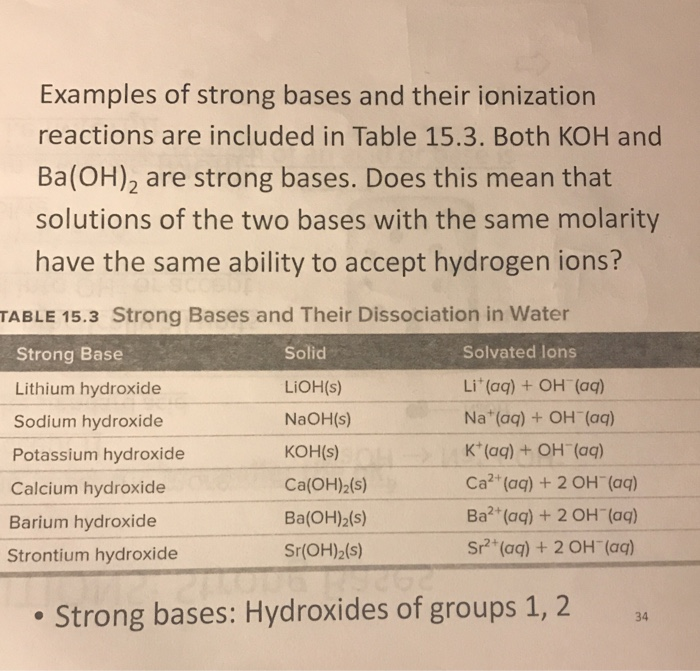
SOLVED:The pOH of a strong base solution is 1.88 at 25^∘ C. Calculate the concentration of the base (a) if the base is KOH and (b) if the base is Ba(OH)2.