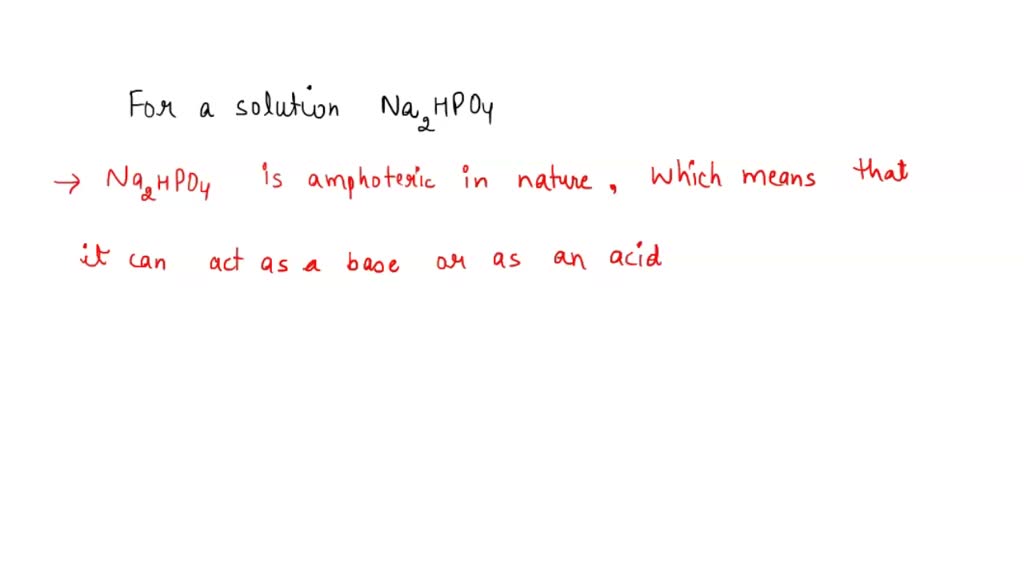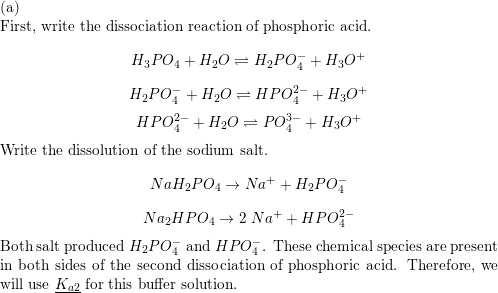OneClass: 2. In the following reactions, label the acid, base, conjugate acid and conjugate base. (4 ...

A buffer solution 0.04 M in Na2HPO4 and 0.02 M in Na3PO4 is prepared. The electrolytic oxidation of 1.0 milli - mole of the organic compound RNHOH is carried out in 100
Q. The equivalent mass of H3PO4 (Molecular weight = 98 g/mol) and Na2HPO4 (Molecular weight = 142 g/mol) in the reaction are respectively : H3PO4 + 2NaOH → Na2HPO4 + 2H2O (1) 49, 142 (2) 49, 71 (3) 98, 71 (4) 98, 142
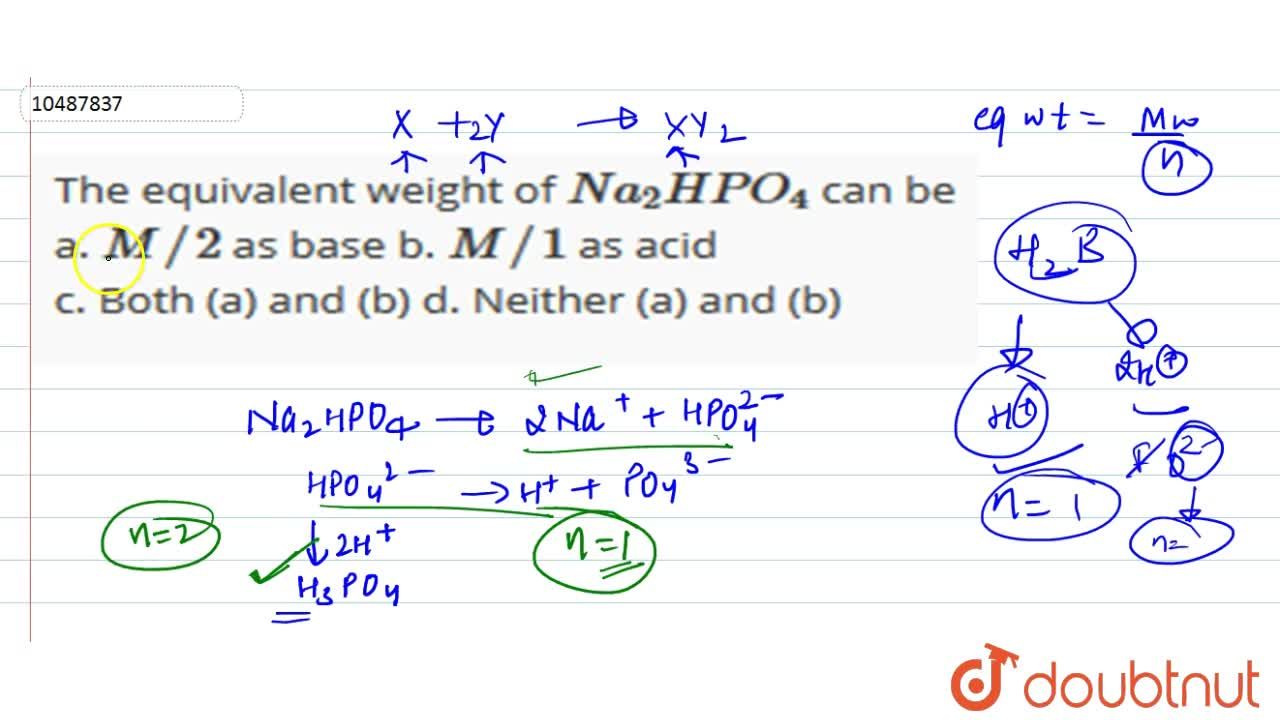
The equivalent weight of Na(2) HPO(4) can be a. M//2 as base b. M//1 as acid c. Both (a) and (b) d. Neither (a) and (b)
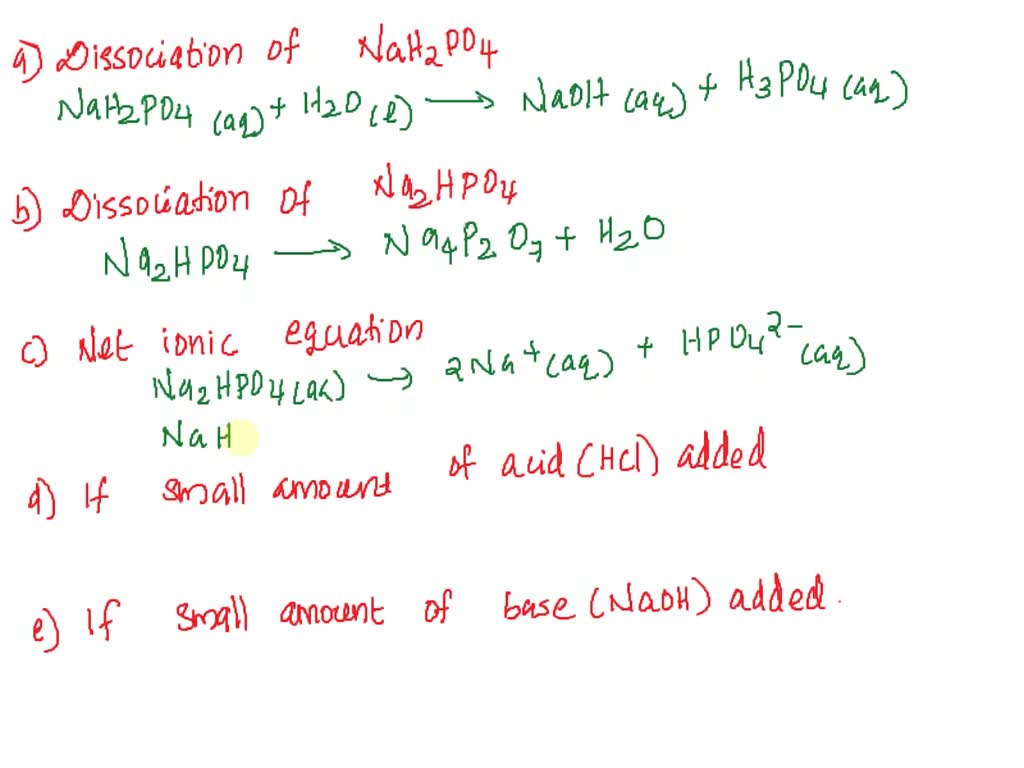
SOLVED: A buffer was made by mixing aqueous solutions of NaH2PO4 and Na2HPO4 together. This buffer is made by mixing two salts together. a. Write the balanced dissociation reaction for solid NaH2PO4

OneClass: Equal molar quantities of sodium hydroxide and sodium hydrogenphosphate (Na2HPO4) are mixed...

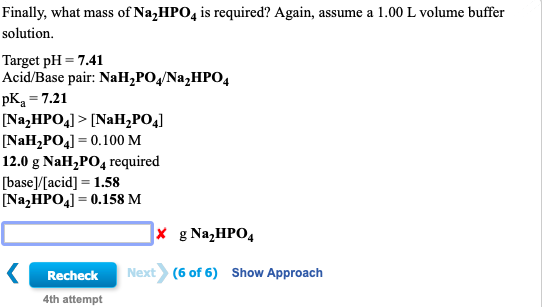
Calculate the pH of a buffer solution obtained by dissolving 25.0 g of KH2PO4(s) and 38.0 g of Na2HPO4(s) in water and then diluting to 1.00 L. | Homework.Study.com
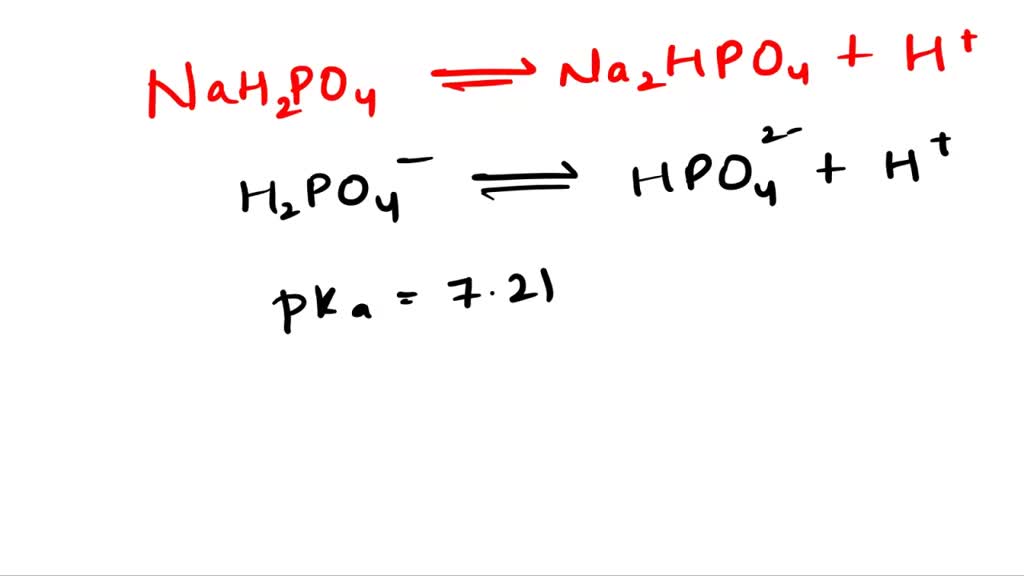
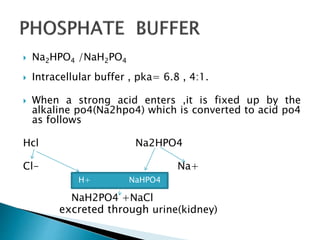
SOLVED: 1. Why is the equilibrium between the acid NaH2PO4, and its conjugate base Na2HPO4, a suitable buffer for maintaining intracellular pH (pH 6.9-7.3)?

![An example of an acid salt is [CH(3)COONa//NaNO(3)//Na(2)HPO(4)//NaKCO(3)] An example of an acid salt is [CH(3)COONa//NaNO(3)//Na(2)HPO(4)//NaKCO(3)]](https://d10lpgp6xz60nq.cloudfront.net/web-thumb/643651395_web.png)