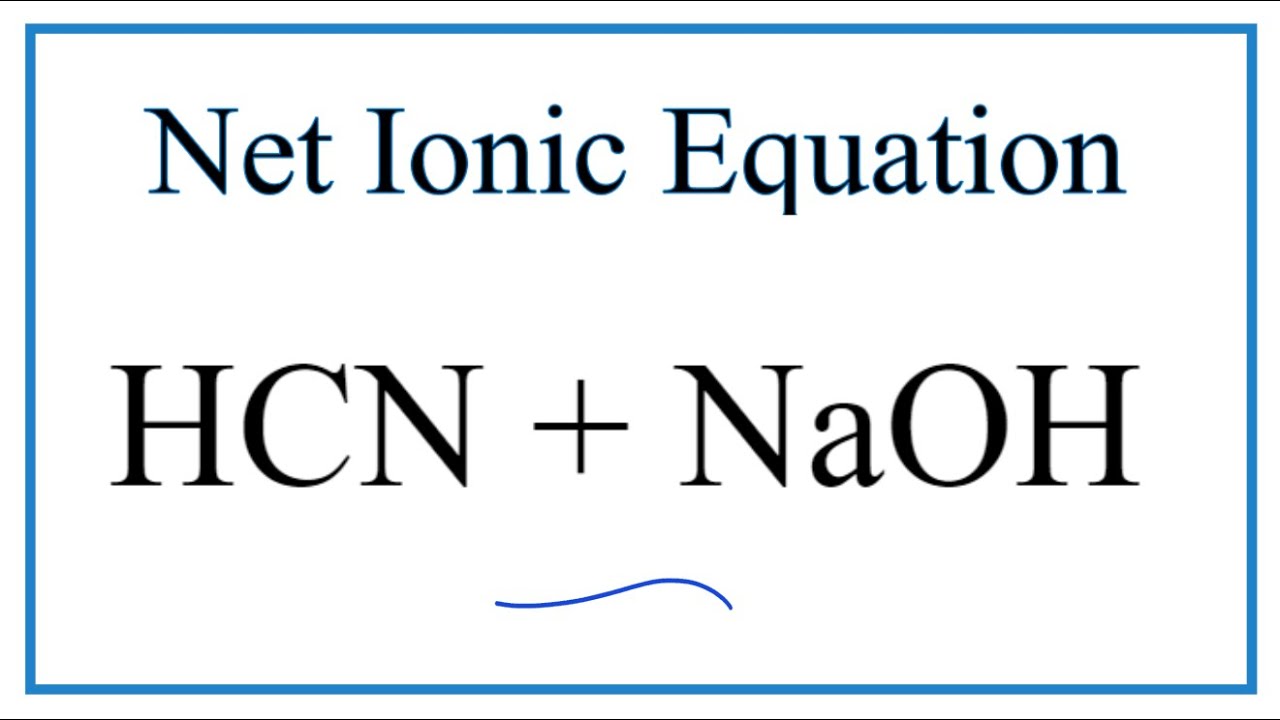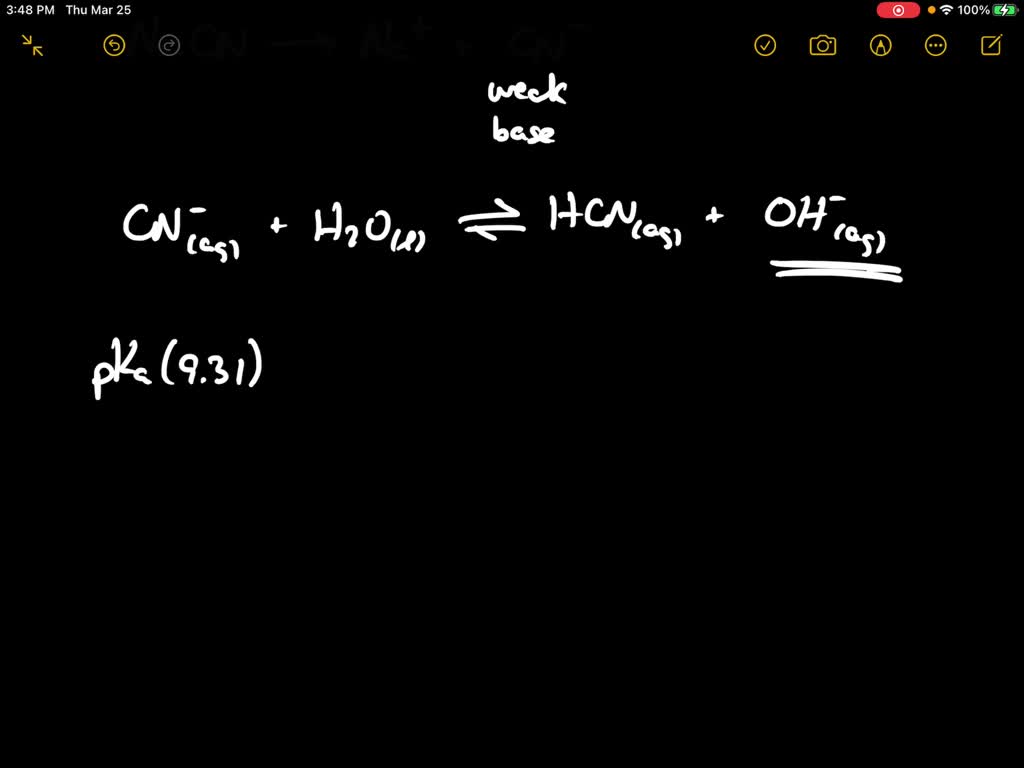
SOLVED:When NaCN dissolves in water, the resulting solution is basic. Account for this observation given that p Ka for HCN is 9.31.

SOLVED: 16- Consider a solution of 2.0 M HCN and 1.0 M NaCN (Ka for HCN = 6.2 10 10). Which of the following statements is true? A) The solution is not

Draw the major product formed in the following reaction with NaCN and other reactants ethanol and water. | Homework.Study.com

Rational Catalysis Design on the Basis of Mechanistic Understanding: Highly Efficient Pd-Catalyzed Cyanation of Aryl Bromides with NaCN in Recyclable Solvents | Journal of the American Chemical Society

Predict if the solutions of the following salts are neutral, acidic or basic: NaCl, KBr, NaCN, NH(4)NO(3), NaNO(2) and KF

Acids Lesson 6 Acid Rain & Hydrolysis. Acid Rain The cause of Acid Rain is the release of acid anhydrides into the environment. Acid Anhydrides are nonmetal. - ppt download

acid base - Why do we need three equations to find the pH of NaCN, given Ka(HCN)? - Chemistry Stack Exchange

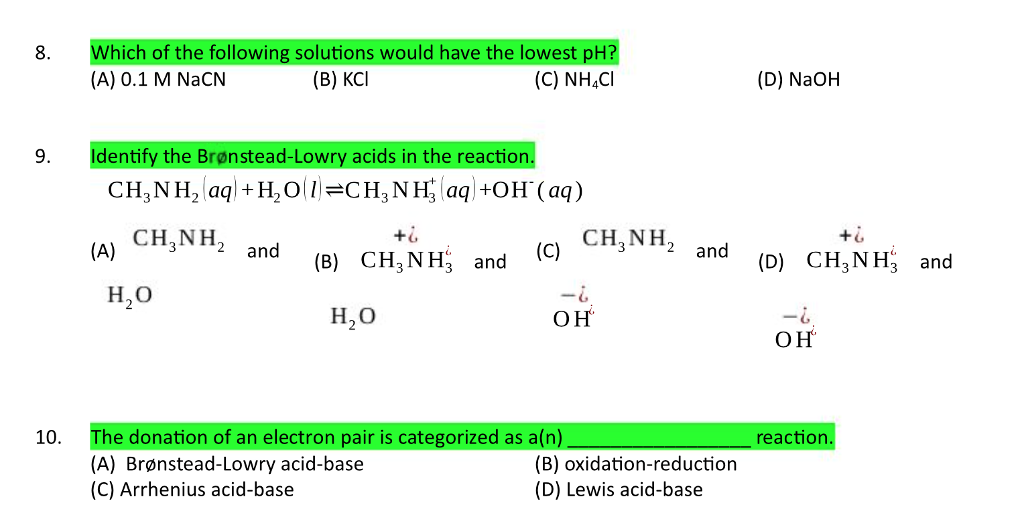
Exam 3 Practice Problems - Sample Problems (Acid/Base and Solubility) CHM Determine if the following - StuDocu