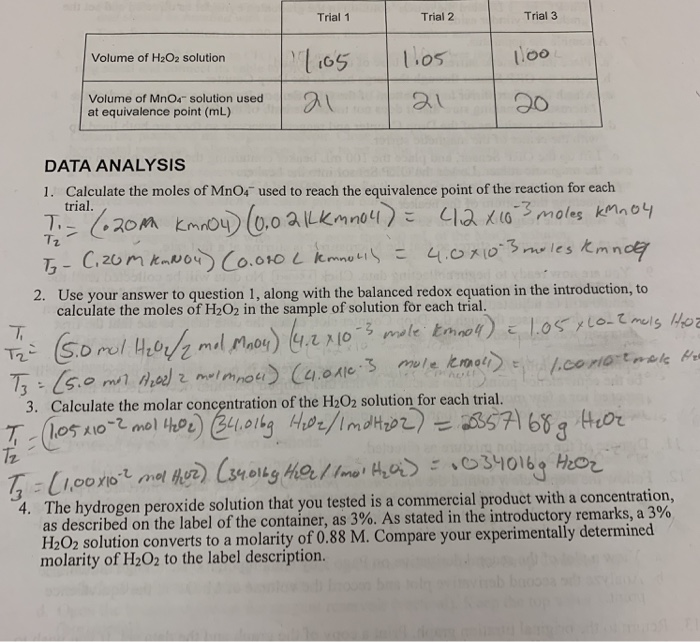
SOLVED: one method of determining the concentration of hydrogen peroxide ( H2O2) in a solution is through titration with the iodide ion. The net ionic equation is H2O2 + 2I-+2H+ –> I2+ 2H2O.
SOLVED: Calculate the final molarity of H2O2 if 5.2 mL of a 3.0% w/w H2O2 solution, which has a density of 1.0 g/mL, is added to 5.2 mL of a starch-iodide solution.
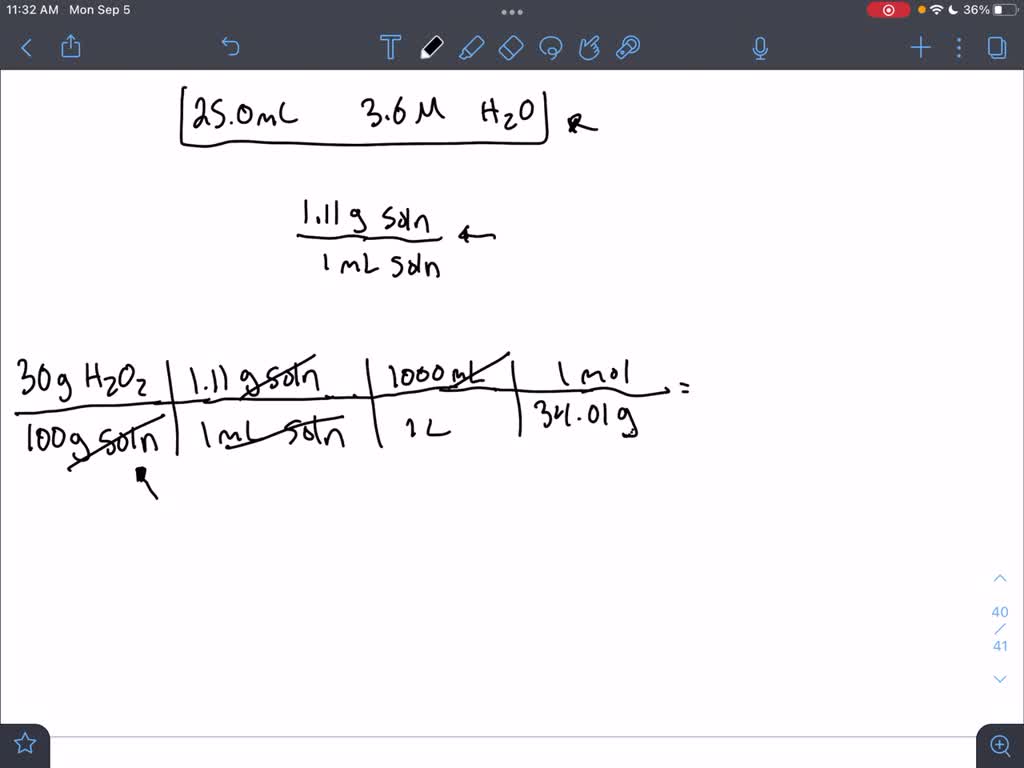
SOLVED: You are required to make up 25.0 ml of a 3.6 M solution of hydrogen peroxide (H2O2). You have at your disposal a stock solution that is 30% by weight, H2O2,
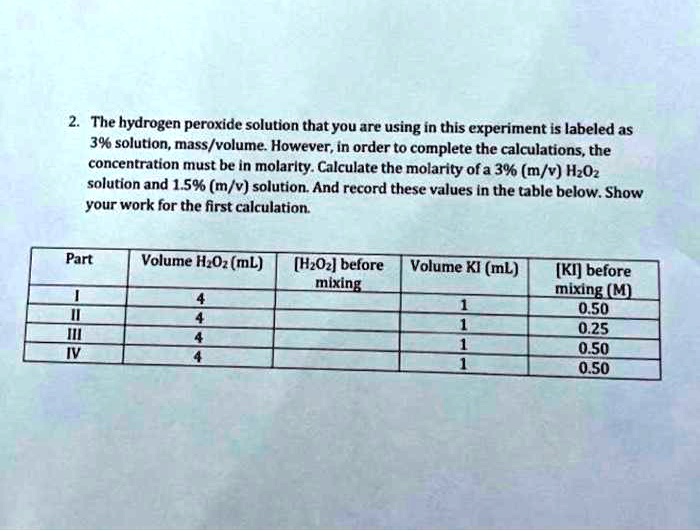
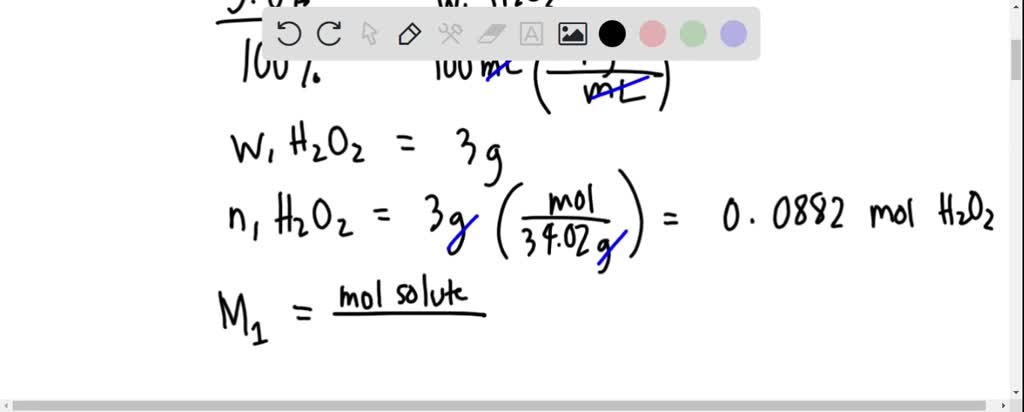
SOLVED: The hydrogen peroxide solution that you are using in this experiment is labeled as 3% solution; mass/volume However, in order to complete the calculations, the concentration must be in molarlty Calculate

Calculate the molarity and molality of a 13% solution (by weight of sulphuric acid). Its density is 1.090 g/ml.

Calculator the equilibrium concentration of gaseous hydrogen peroxide over solution – Lukáš Kolík – physico-chemical calculation – working a personal webpage
Welcome to Chem Zipper.com......: Calculate the Molarity of H2O2 if 11.2 ml H2O2 require 30 ml of 0.5 M K2Cr2O7 for its Oxidation . also calculate the volume of strength of H2O2.

Concentration of Hydrogen Peroxide in a 10 Volume Solution - An Interesting Stoichiometry Problem - YouTube


.gif)