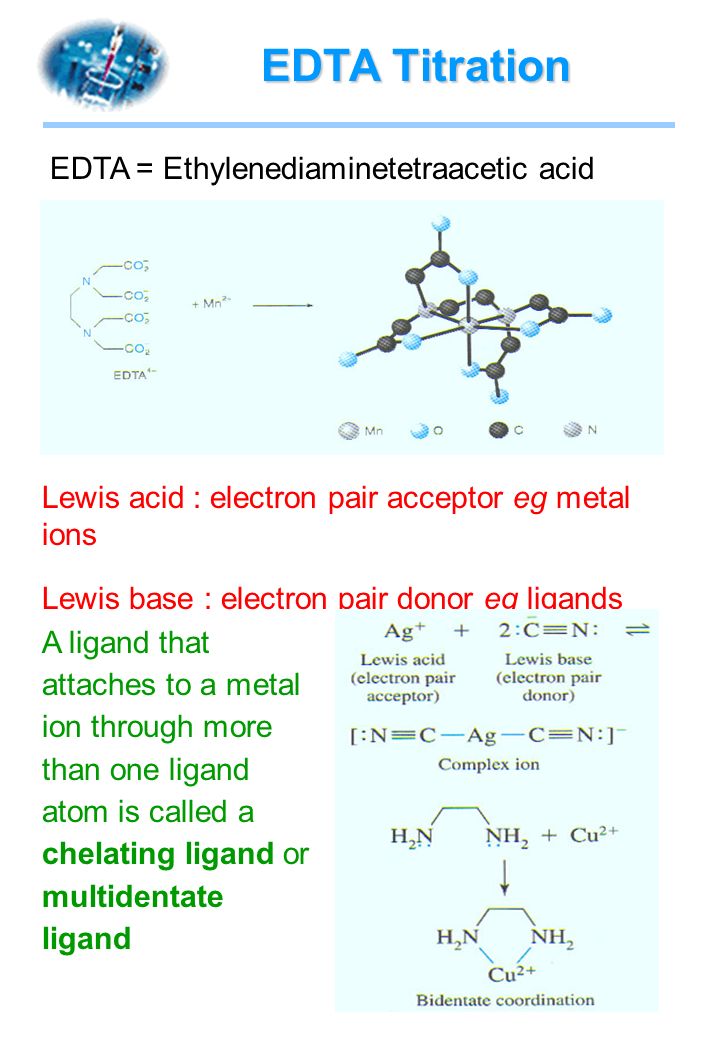
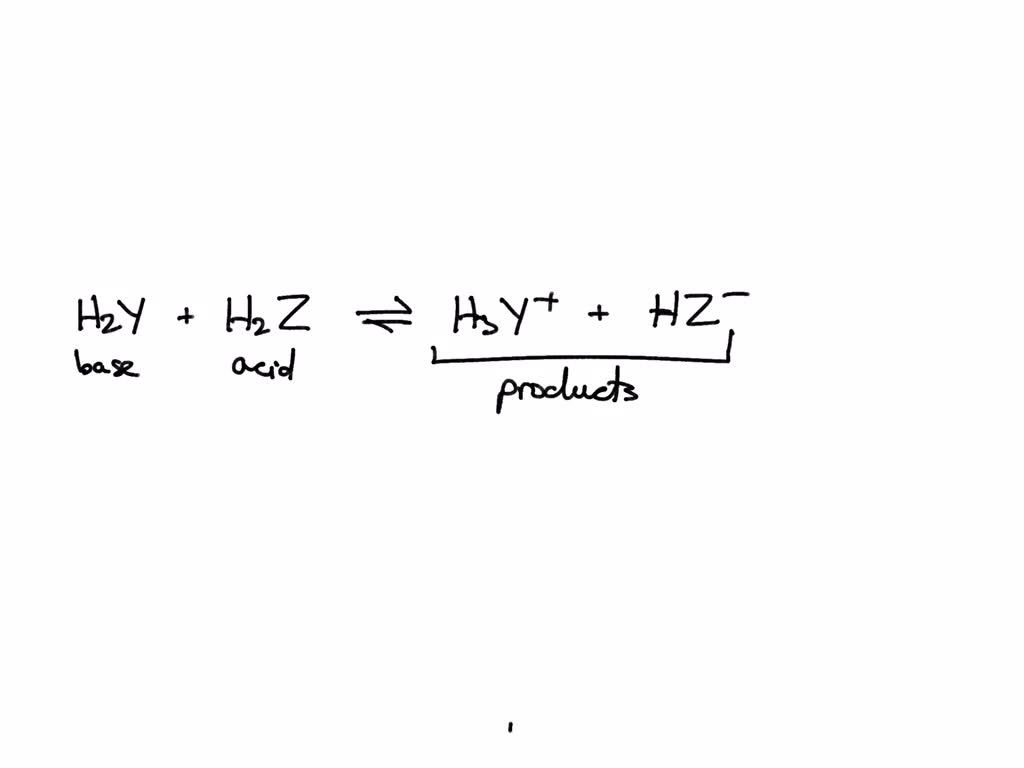Complete a net ionic equation for each proton-transfer reaction using curved arrows to show the flow of electron pairs. Write Lewis structures for all starting materials and products, label the original acid

Highly Selective Surface Lewis Acid−Base Reaction: Trimethylamine on Si(100)c(4×2) | The Journal of Physical Chemistry B
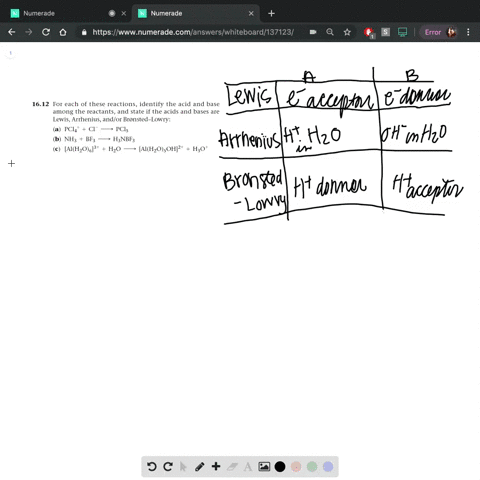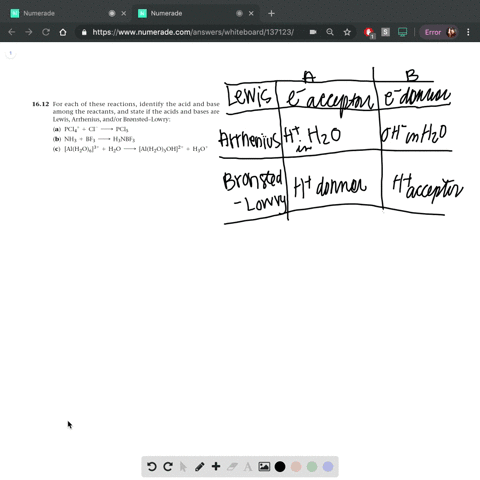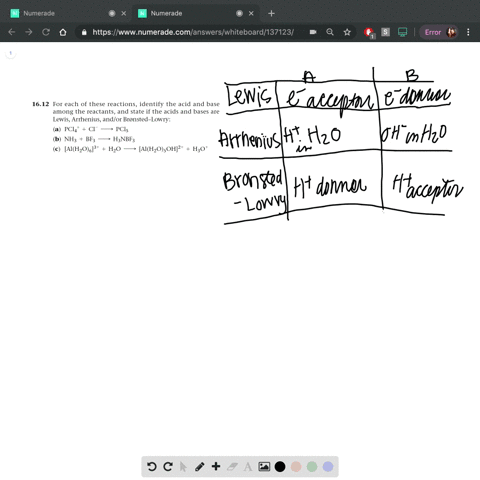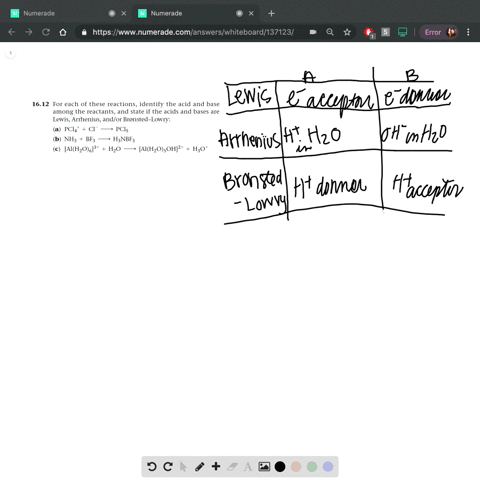
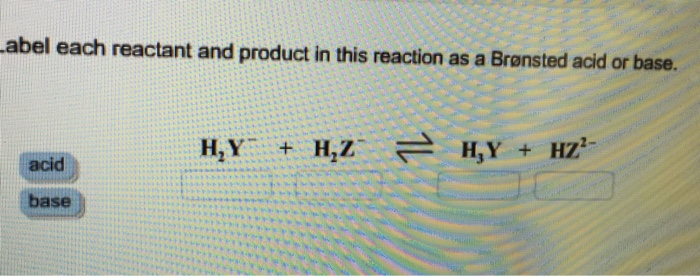
SOLVED: In the reaction between H2Y - and H2Z - , if H2Y - acts like a base, and H2Z - acts like an acid, the products of the reaction are: A)
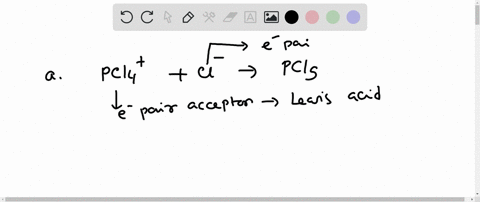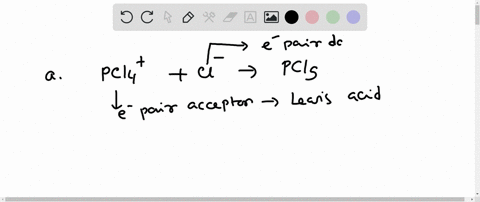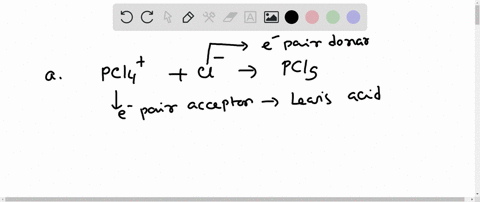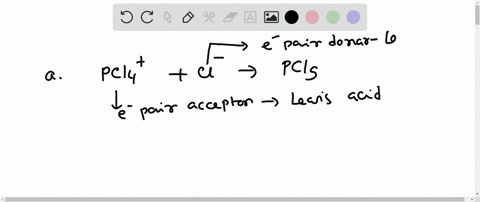
SOLVED: In the reaction between H2Y - and H2Z - , if H2Y - acts like a base, and H2Z - acts like an acid, the products of the reaction are: A)

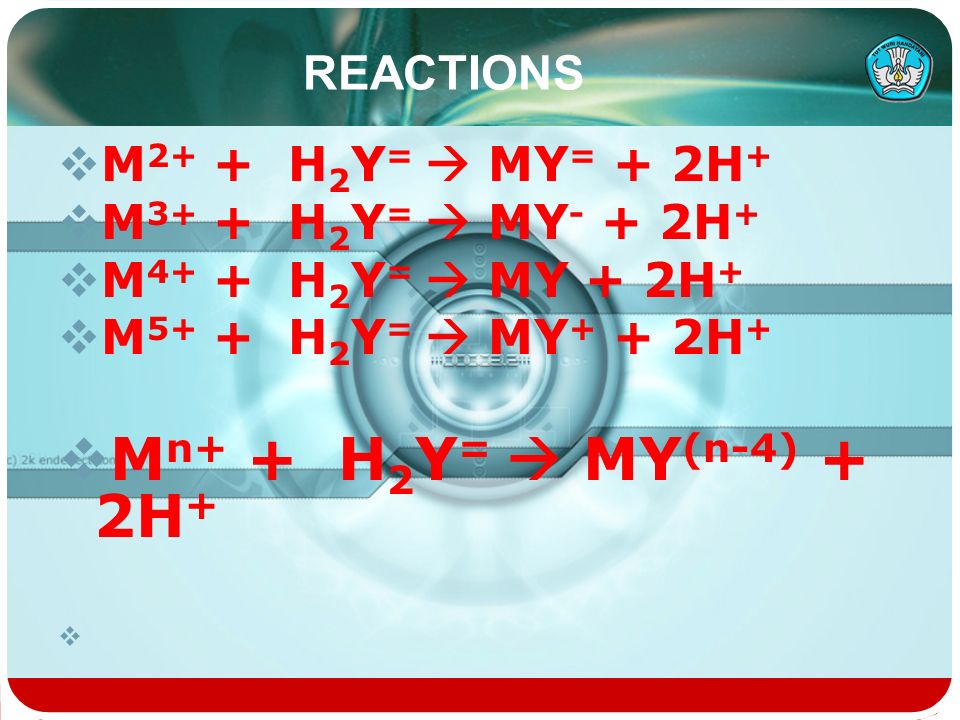
SOLVED: 30.At what pH would you expect the aminoacid prolinePto be fully protonated?pK=1.952,pK2=10.640 A.1.952 B.1.750 C.2.500 D.10.640 E.7.000 31.Given pKvalues of citric acid2-hydroxypropane-1,2,3-tricarboxylic acid,pK=3.128,pK=4.761,and pK3= 6.396 ...

Complete the equation for the reaction between the following Lewis acid-base pair. Label which starting material is the Lewis acid and which is the Lewis base, and use curved arrows to show

Complete the equation for the reaction between the following Lewis acid-base pair. Label which starting material is the Lewis acid and which is the Lewis base, and use curved arrows to show

The aqueous solution of transition metal salt changes colour from pink to blue, when concentrated hydrochloric acid is added to it. The change in colour is due to

acid base - How to predict the color of a pH indicator using Le Chatelier's principle - Chemistry Stack Exchange

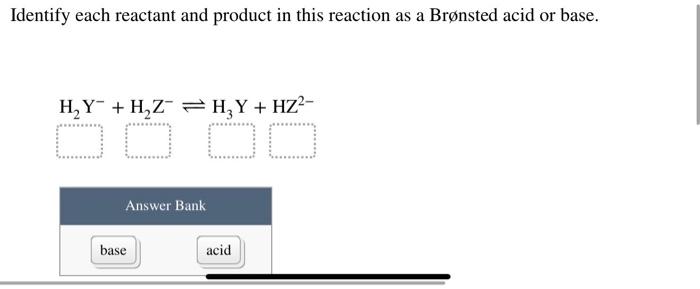
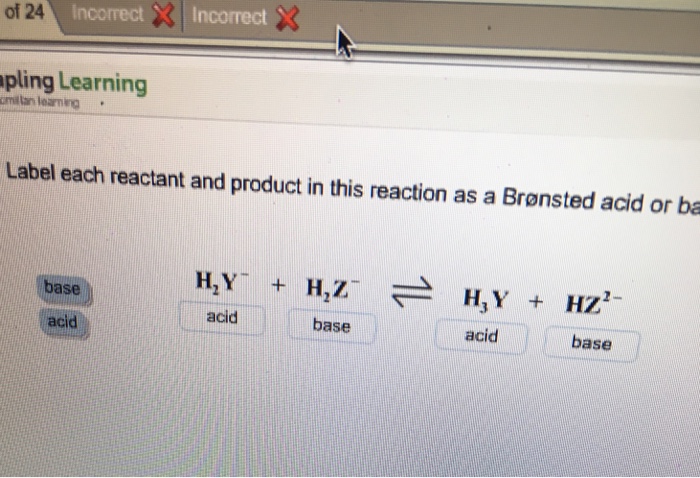
SOLVED: Identify each reactant and product in this reaction as a Bronsted acid or base. Hz Y- + HzZ acid base H; Y + HZ2 - base acid Answer Bank base acid

MEP maps of isolated TrHX, TrH2X and H2Y molecules (Tr = Ga, In; X = F,... | Download Scientific Diagram