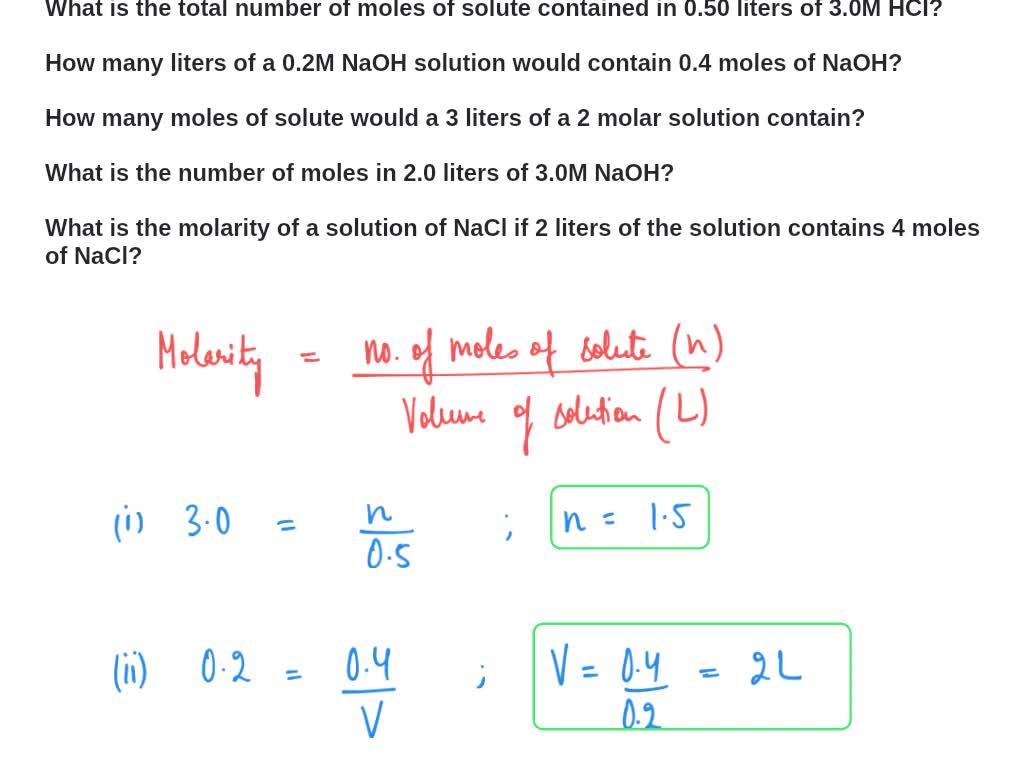
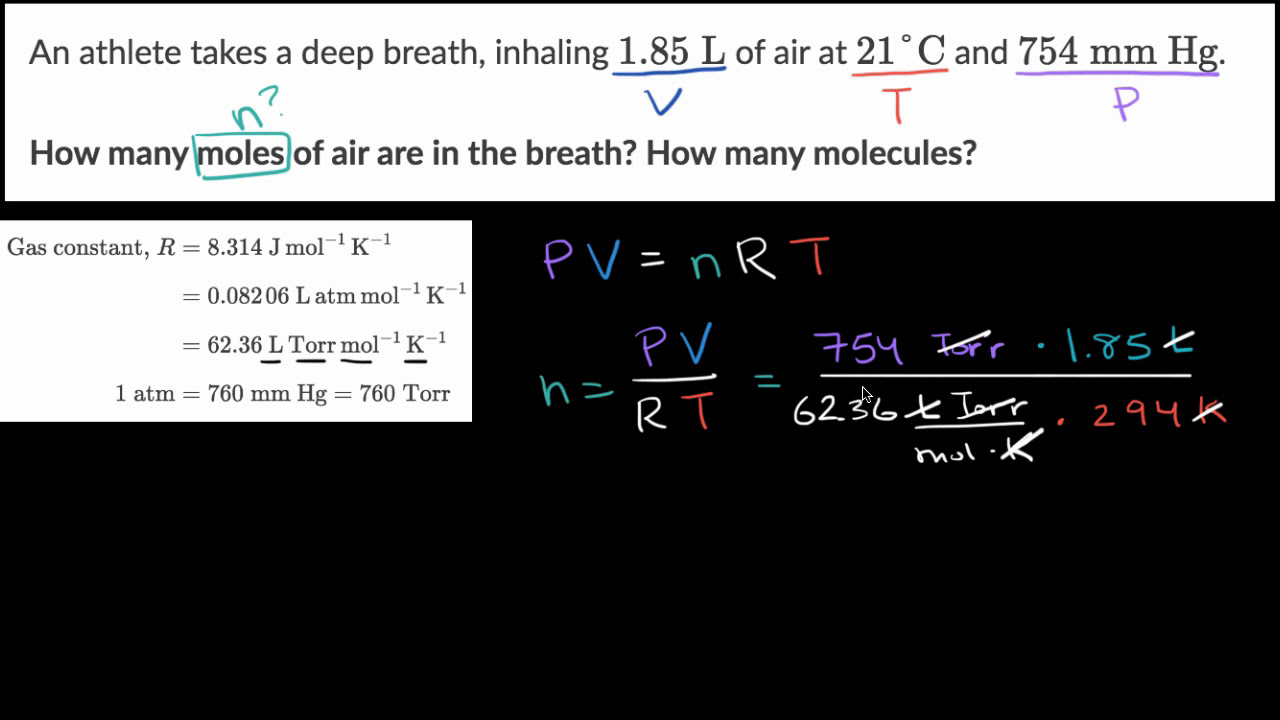
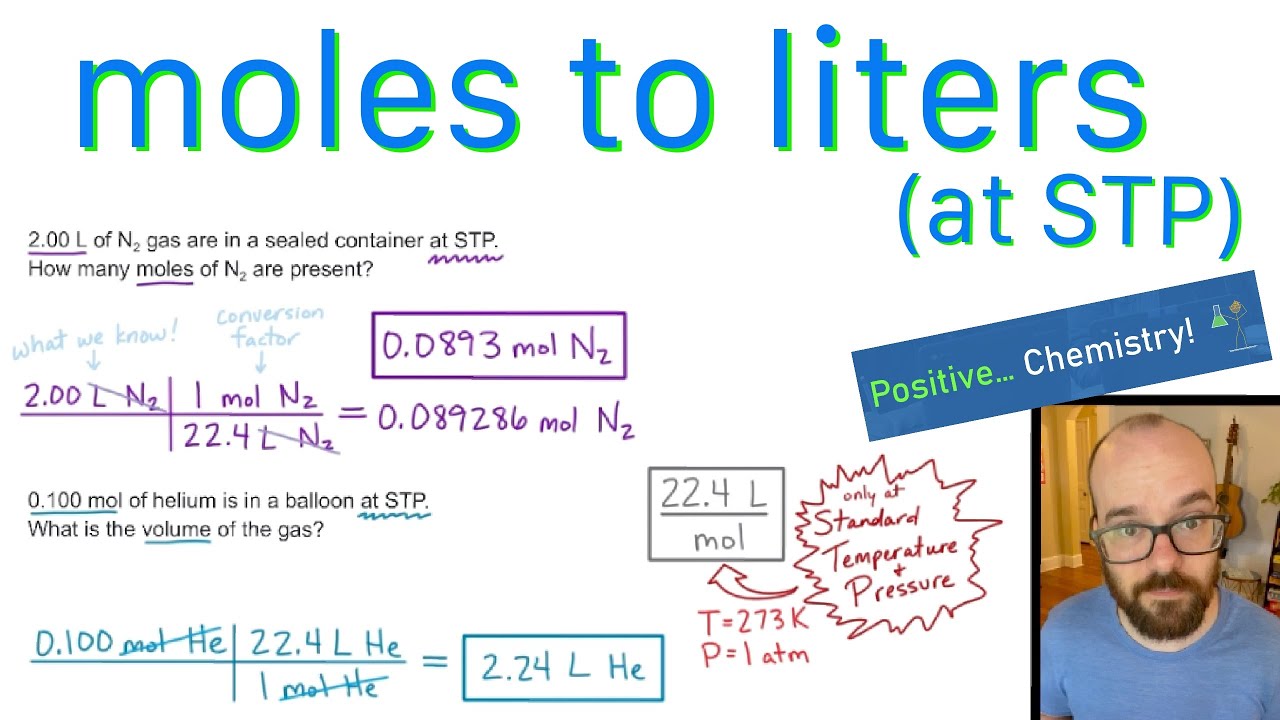
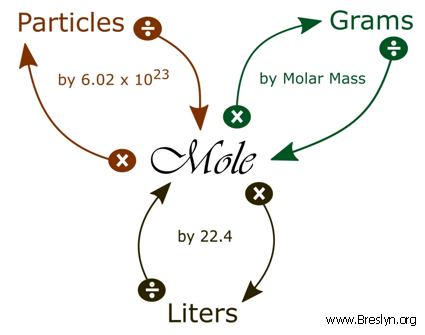
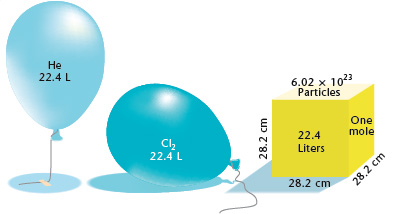
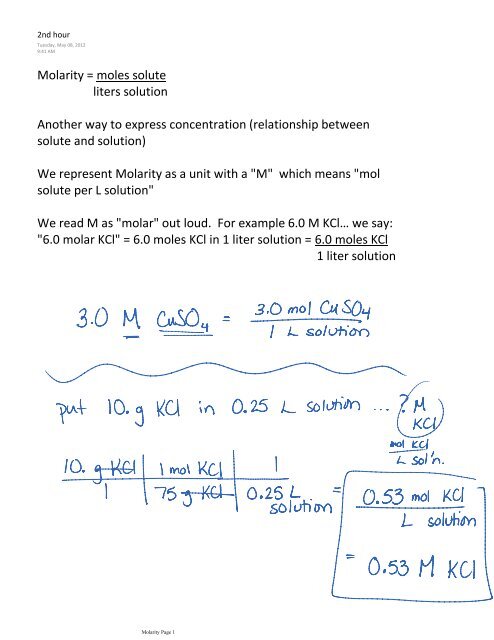
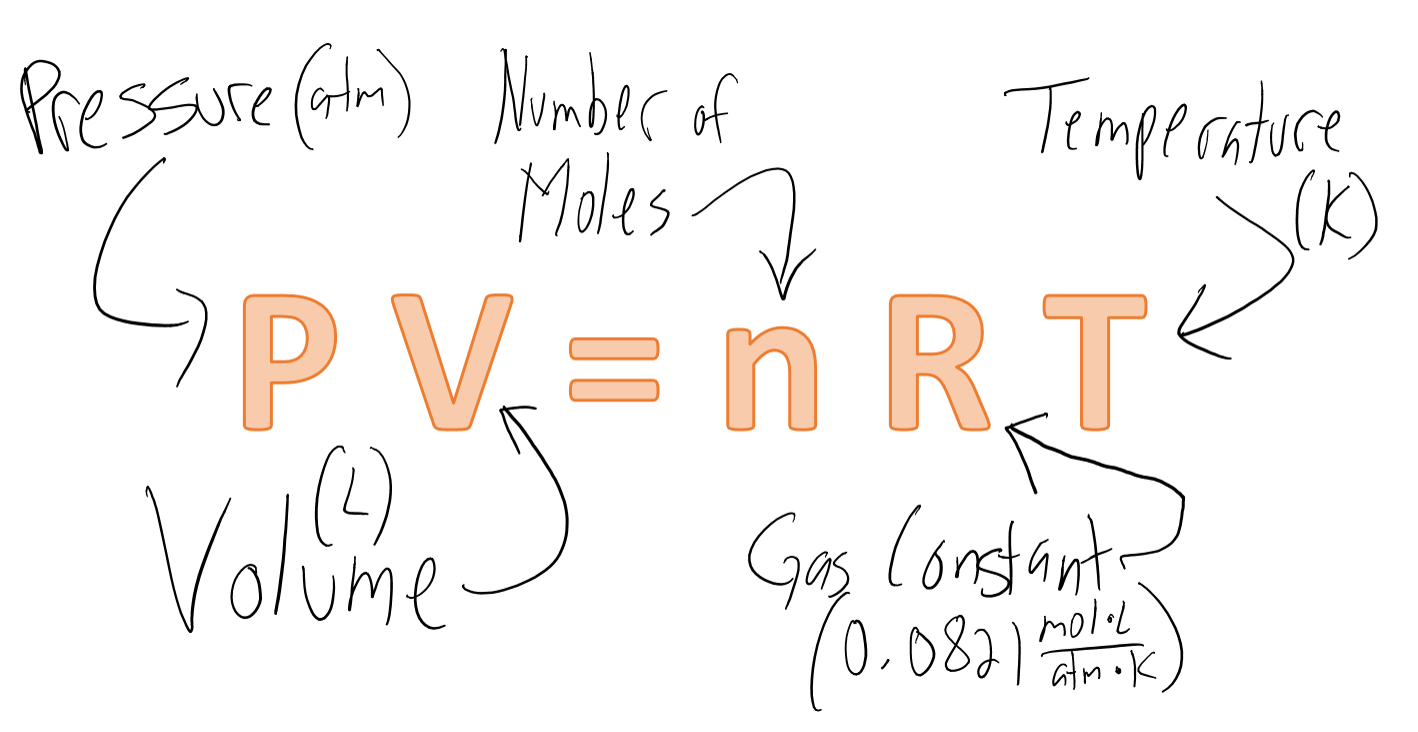Answer Key Moles & Liters volume .docx - Moles & Liters Volume We've established that it's not practical to count atoms so we often need to use | Course Hero
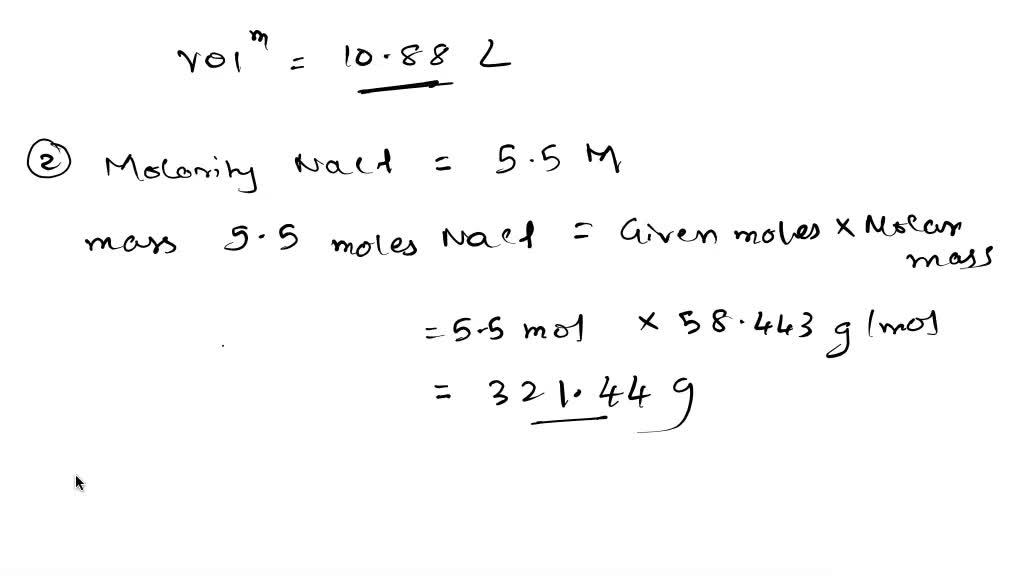
SOLVED: How many liters of a 0.114 M NaOH solution contains 1.24 moles of NaOH? The concentration of an NaCI solution (in water) expressed as Molarity is 5.5 M Express this concentration

Review: Mole Conversions: Convert 3 mols Oxygen to grams: Convert 42 grams Chlorine to mols: What is % composition? What is the %comp of magnesium in magnesium. - ppt video online download