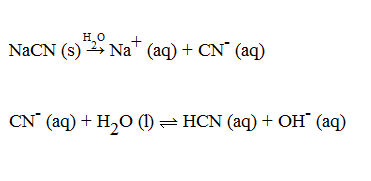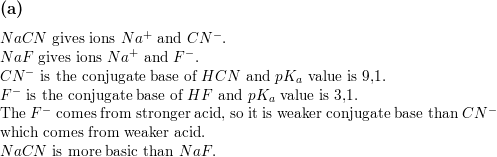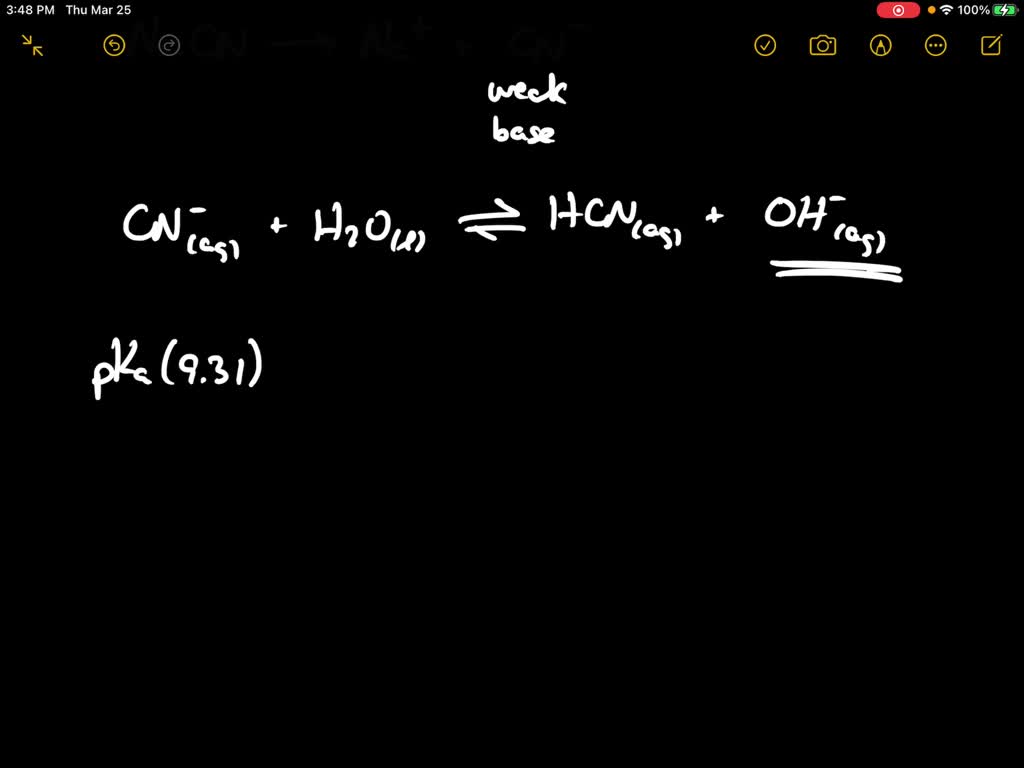
SOLVED:When NaCN dissolves in water, the resulting solution is basic. Account for this observation given that p Ka for HCN is 9.31.

Rational Catalysis Design on the Basis of Mechanistic Understanding: Highly Efficient Pd-Catalyzed Cyanation of Aryl Bromides with NaCN in Recyclable Solvents | Journal of the American Chemical Society
✓ Solved: An unknown salt is either NaCN, NaC2H3O2, NaF, NaCl, or NaOCl. When 0.100 mole of the salt...

Draw the major product formed in the following reaction with NaCN and other reactants ethanol and water. | Homework.Study.com

Predict if the solutions of the following salts are neutral, acidic or basic: NaCl, KBr, NaCN, NH(4)NO(3), NaNO(2) and KF
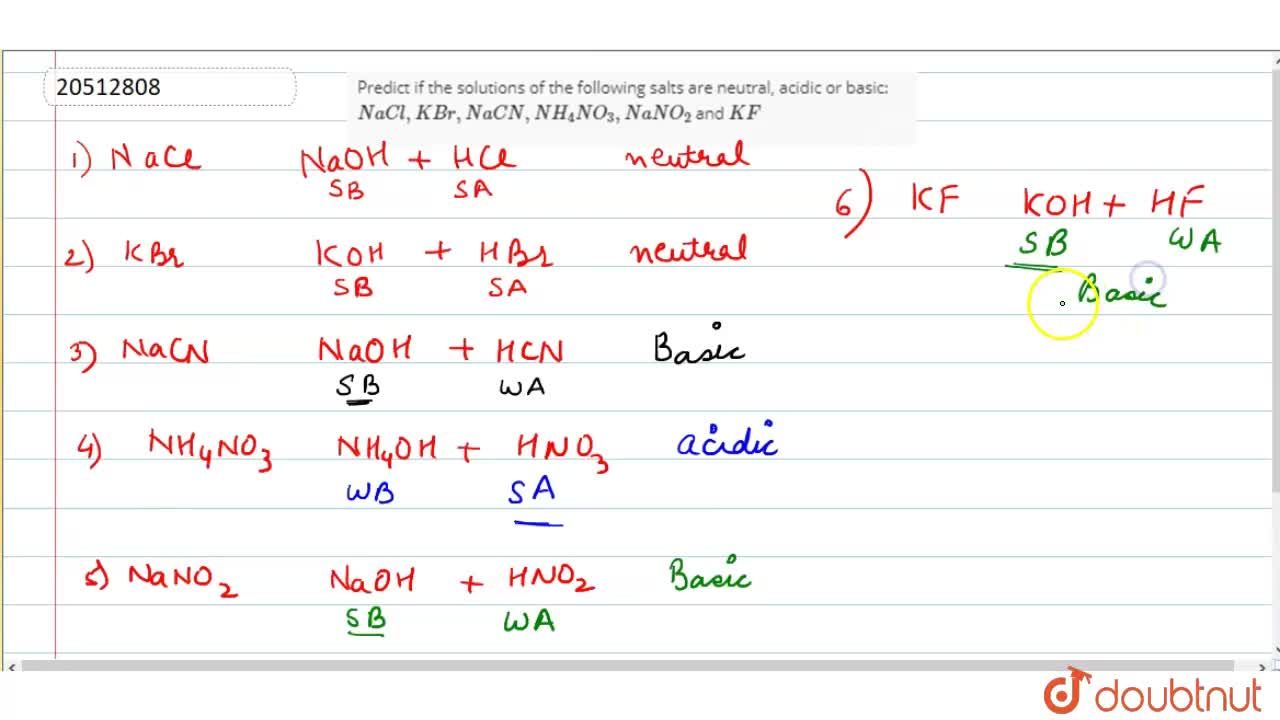
Predict if the solutions of the following salts are neutral, acidic or basic: NaCl, KBr, NaCN, NH(4)NO(3), NaNO(2) and KF

acid base - Why do we need three equations to find the pH of NaCN, given Ka(HCN)? - Chemistry Stack Exchange
What is the % hydrolysis of NaCN in N/80 solution when dissociation constant for HCN is 1.3 x 10^-9 & Kw = 1 x 10^-14 - Sarthaks eConnect | Largest Online Education Community